Now that you’ve been diagnosed with non-small cell lung cancer (NSCLC), it’s important to talk to your oncologist about the benefits of genomic testing. Genomic testing refers to biomarker testing of your tumor (e.g. the tumor’s genetic makeup). Immediately testing your cancer cells can help your care team determine the most appropriate treatment path, and evaluate your qualifications for clinical trials regarding new drug therapies that might help you, too.
Here are some starter questions about testing to discuss with your oncologist:
Why should I pursue cancer genomic testing?
Over the last few years, oncologists have turned to a more personalized approach to medicine, where biomarker testing for individual tumors is becoming a routine part of the diagnosis and staging for NSCLC patients around the country.
How many mutations are there?
At Memorial Sloan Kettering, they screen NSCLC patients for mutations in more than 300 genes at once. Most of the genetic changes are found in cancer cells, which means they’re not hereditary and can’t be passed on. These mutations include:
- EGFR, an abnormal gene found in 10% of NSCLC patients and 50% of never-smokers with lung cancer
- KRAS, a mutation found in 25% of NSCLC patients
- ALK, an abnormal gene found in 5% of NSCLC patients and 10-15% of never-smokers with lung cancer
How can testing affect my treatment plans?
Genomic testing will determine exactly which mutations are present in your cancerous tumor, which in turn might qualify you for a recently approved biomarker-driven treatment, or a clinical trial for a new drug therapy. If testing determines that your cancer might return after surgery, your oncologist might recommend a different course, such as chemotherapy, radiation, or targeted therapy. SOURCE, SOURCE
How does testing work?
When it comes to genomic testing of your tumor for specific biomarkers, your team can typically rely upon the same tissue sample derived during your original tumor biopsy.
After your initial pathology analysis, your oncologist will refer you for one of the following tests:
- Fluorescence in situ hybridization (FISH), where a pathologist adds your DNA to a tissue sample to look for chromosome binding under a fluorescent microscope.
- Immunohistochemistry, a staining technique where human-made antibody proteins are added to your cancer cells.
- Next-generation sequencing(NGS), where the DNA sample is run through a machine to verify genetic sequencing.
Is genomic testing recommended for everyone?
World-renowned treatment centers like Memorial Sloan Kettering Cancer Center use testing every day to help cancer patients understand the long-term effects of their diagnosis and weigh the benefits of clinical trials, surgery, radiation, and other therapies. SOURCE
What if I choose not to move forward with testing?
Decisions after your diagnosis are up to you and your care team. With the rise of precision medicine and the continued evolution of biomarker-driven treatments, doctors will continue to have more tools on hand to determine what course of treatment might be best for you. Testing will ensure you’re not missing out on any treatment options that could influence your care.
If you authorize your medical records within the Outcomes4Me app, our team of oncology nurse practitioners can alert you to genomic testing you should consider.
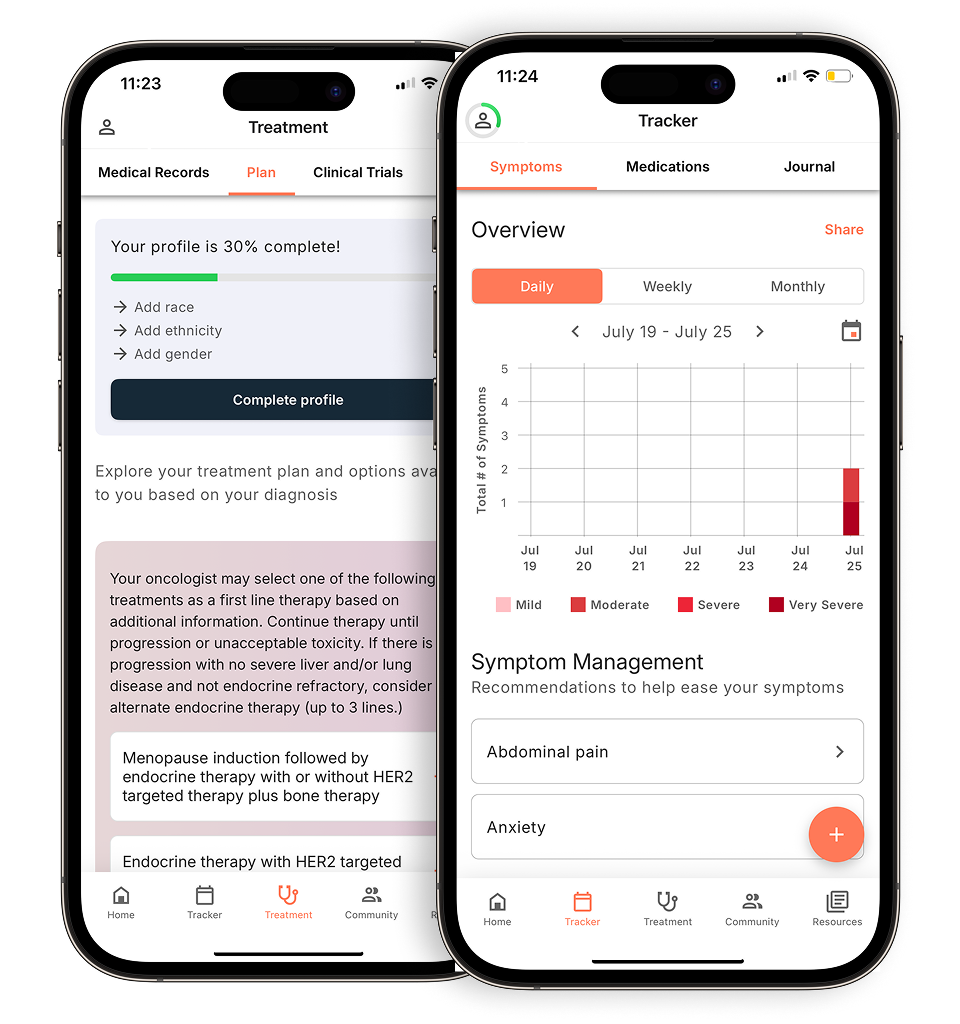
Personalized support for real care decisions
Understand your diagnosis, explore clinical trials, and track symptoms--all in one place.
Get started
Compare treatments, prepare for appointments, and track side effects—all in the app
Built for your diagnosis, Outcomes4Me gives you the tools to make confident, informed decisions—right when you need them.
Continue in app